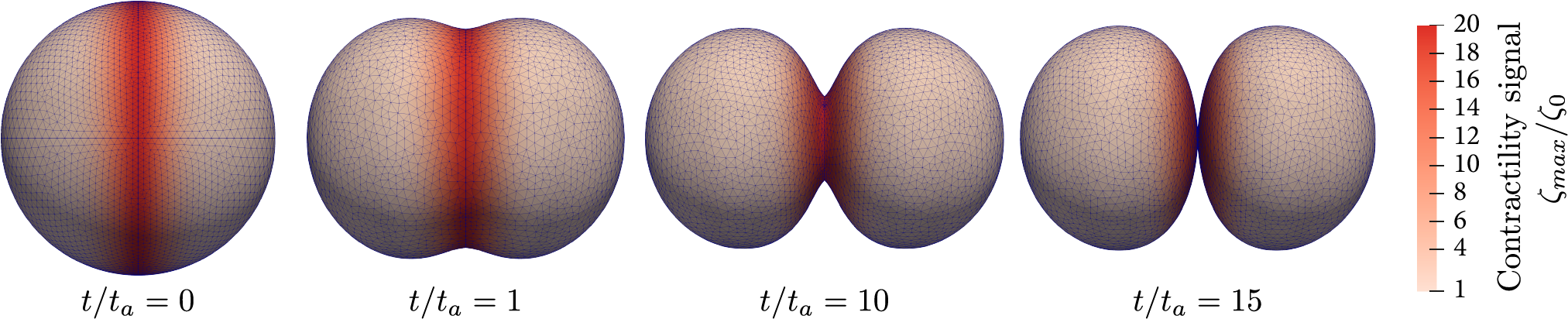RESEARCH
How do cells form embryos and tissues?
Embryos and tissues are highly complex systems, made of interacting autonomous agents, the cells, that have evolved to robustly assemble into tissues and organs.
In spite of huge progress in molecular genetics and live imaging, we know little on how cells self-organize into an embryo or tissue from a single cell.
“How do cells control their shape, position and fate ?
What information is processed by a cell to take its next decision ?
How is morphogenetic information transmitted within the embryo ?
What principles underly robustness in early embryo development ?”
To answer the above questions, we follow the strategy below:
We aim at integrating the various spatiotemporal scales underlying early embryogenesis.
This multiscale approach will be key to ultimately relate predictive models of embryos to quantitative experiments.We build novel methods to reverse-engineer from data the minimal feedback controls between biochemical signaling and mechanics.
Our goal is to integrate such feedbacks in realistic 3D simulations to recapitulate multicellular self-organization.We study simultaneously several distant species : mammalian, nematodes, ascidian, cnidarian and annelid embryos.
Thereby, we expect to largely contribute to the emergence of a new field: evolutionary morphogenesis.
1. Multiscale physics and mechanics of multicellular morphogenesis
Morphogenesis couples multiple spatial and temporal scales. Since the tools available to perturb cellular processes operate mainly at the molecular level (drugs, (opto)genetics…), a critical step to better relate experiments to quantitative models of embryo morphogenesis is to characterize how molecular players control the coarse-grained physical properties of cells. This represents a tremendous technical and theoretical challenge. Below a few examples of the topics that we are investigating in this direction.
Collaborations: O. Weiner (UCSF), G. Charras (UCL)
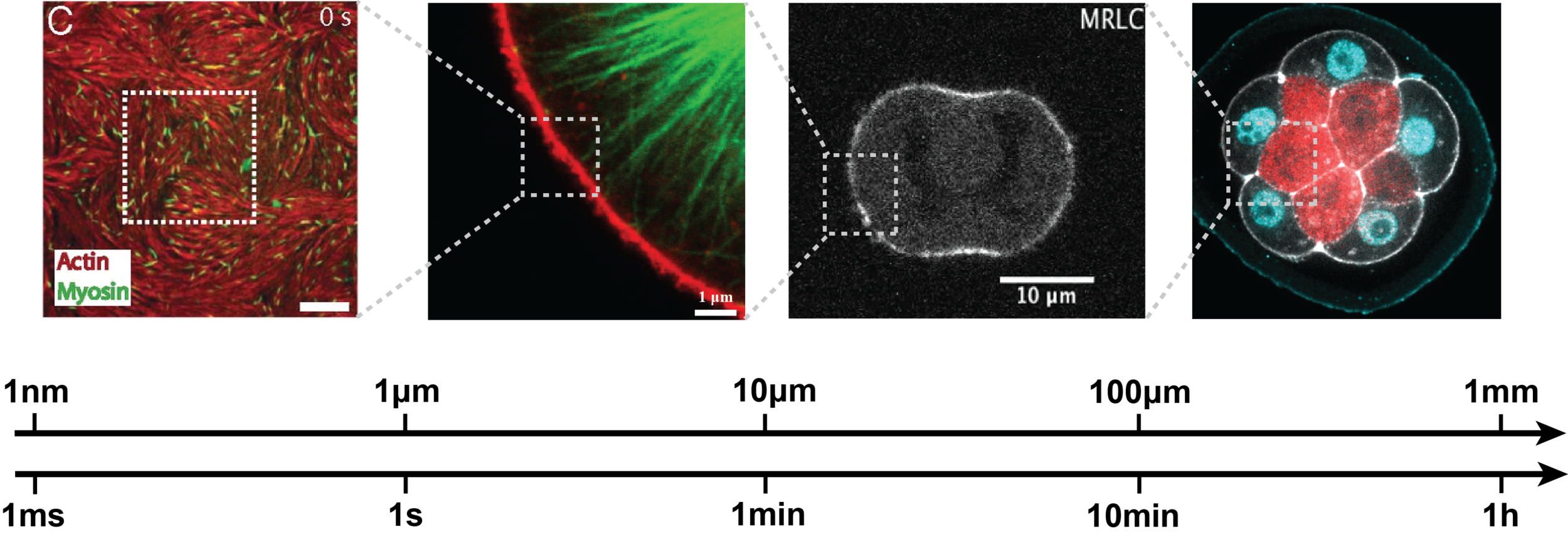
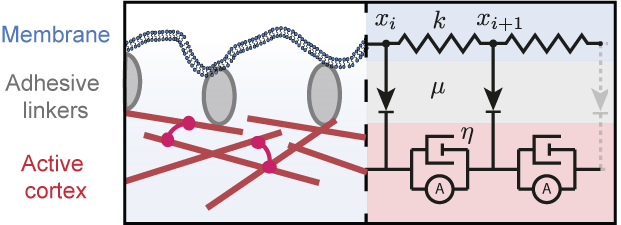
Physics and mechanics of the cell SURFACE
The surface of cells is composed of the plasma membrane and the actomyosin cortex, coupled mechanically through membrane-cortex attachment proteins (MCA). The cortex is a thin layer of polymeric actin filaments, that generates most surface forces shaping embryonic cells. At the mesoscopic level, active gel hydrodynamic theories have proven their efficiency in capturing the essential physics of various actomyosin-based cell processes. But the complex rheological features of the cortex remain poorly understood, in particular in relation to its microscopic constituents, and the mechanical coupling between the membrane and the cortex remain poorly characterized. Combining physical models, agent-based numerical simulations and mathematical downscaling methods , we aim at describing theoretically the membrane-cortex composite mechanics up to the embryonic scale
Collaborations: A. McDougall & R. Dumollard (LBDV),
A. Carvalho (i3S Univ Porto)
Cell division in embryos and tissues
We are working on the modeling of cell division in multicellular context. The current topics that we are investigating are the role of confinement, asymmetric division and the determinants of cleavage patterning in early embryos.
Turlier et al. Biophysical Journal 2014
Maître, Turlier et al. Nature 2016
Borja, Bleyer & Turlier JMPS 2022
Hydraulic and osmotic control of development
The roles of hydraulic and osmotic fluxes processes have been largely overlooked. We are combining hydrodynamics and physical modeling to understand the formation of biological cavities (also called lumens) in embryos and tissues. We aim at integrating osmotic volume control with cell mechanics in 3D realistic simulations.
Dumortier et al. Science 2019
Le Verge-Serandour & Turlier, PLoS Comp Biol 2021
Le Verge-Serandour & Turlier, Sem Cell Dev Biol 2021
MECHANICS OF tISSUES in 3D
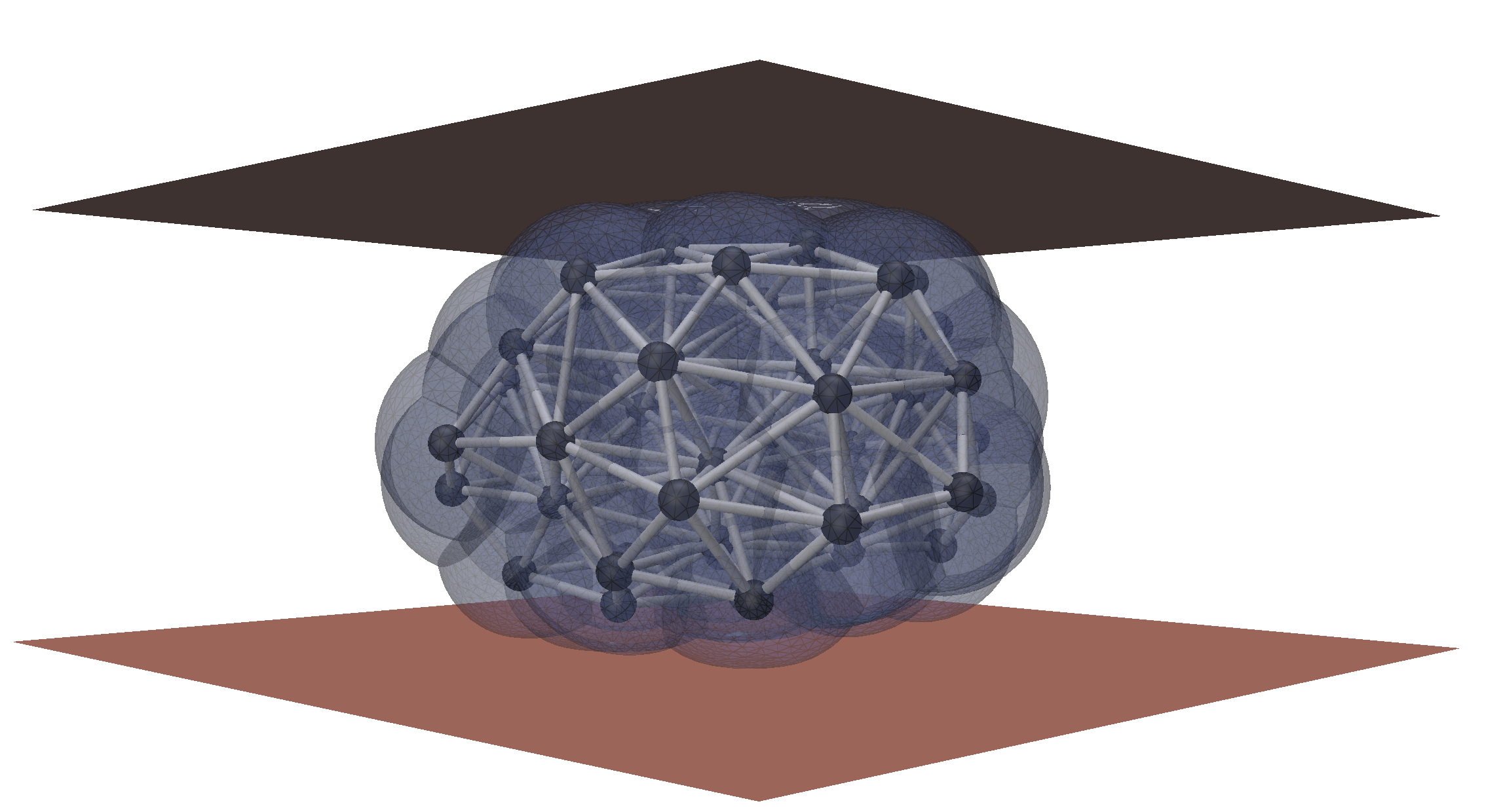
We want to understand how single cell mechanics determines the response of multicellular aggregates, including physiological tissues and organoids. To do so, we use cell-based 3D simulations as in silico experiments to develop continuous rheological models of tissues.
We also develop 3D active nematic continuum theories of tissues, allowing us describing the complex interplay
between cell orientation, tissue stress and shape.
Collaborations: J-L. Maître (Institut Curie)
2. Reverse-engineering development combining physics and data-driven approaches
Embryogenesis is archetypal of a self-organization process, where the emergence of a complex structure stems from the interaction of its elementary parts. To reverse-engineer the minimal feedbacks underlying embryo development, we are working on:
The development of a new theoretical framework that considers cells as intelligent agents, able to process environmental information and take autonomous actions. Integrating prior biological and physical knowledge, this framework should encode cell’s behavior to simulate developmental strategies at the embryo level and to formulate novel experimentally testable hypotheses.
The design of novel data-driven statistical methods (i) to automate the extraction of relevant biophysical information from microscopy and omic data, (ii) to disentangle the complexity of cells behaviour and interactions (iii) to evaluate the robustness and variability of developmental strategies.
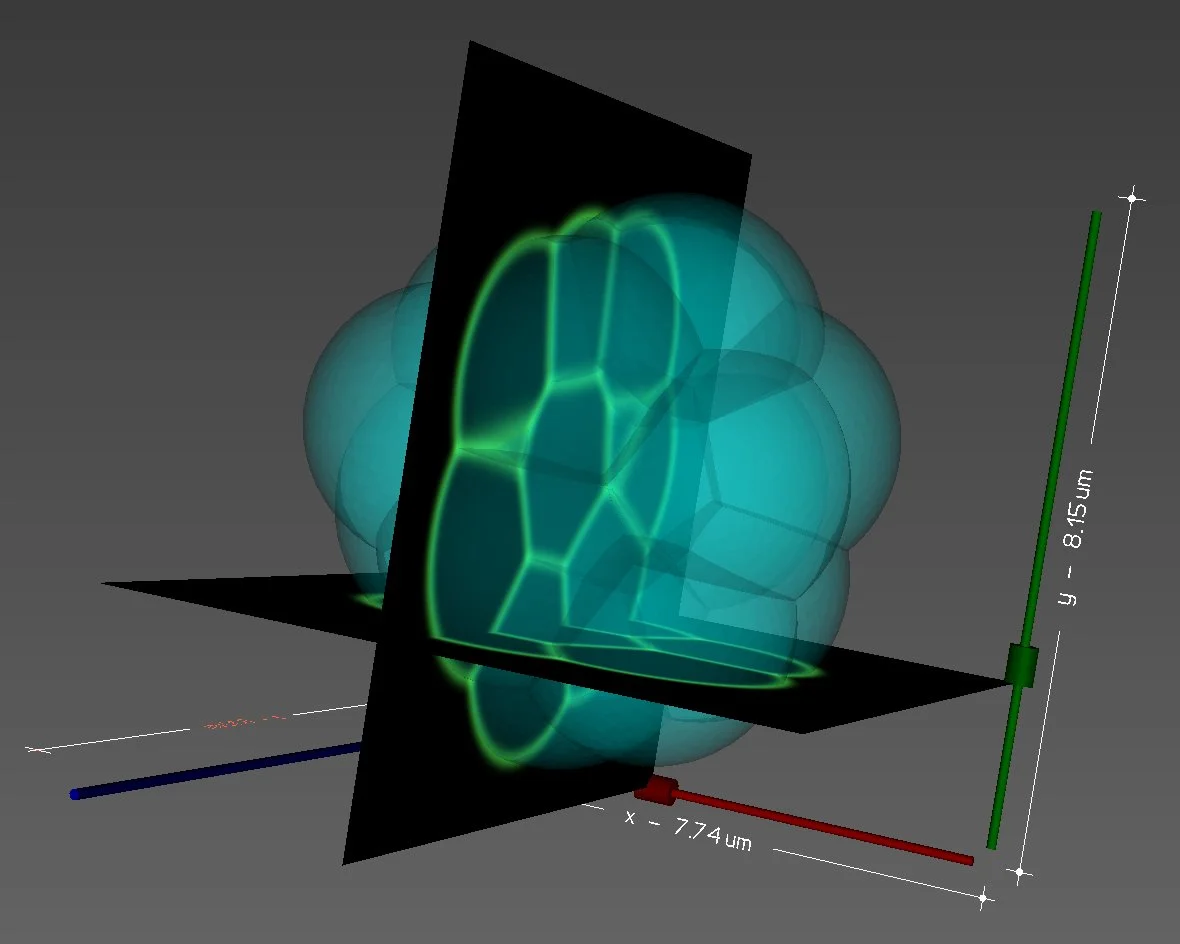
MICROSCOPY Image SEGMENTATION and artificial image generation
The segmentation of microscopy images is one major and recurrent challenge in quantitative biology.
We developed novel mathematical and computational methods:
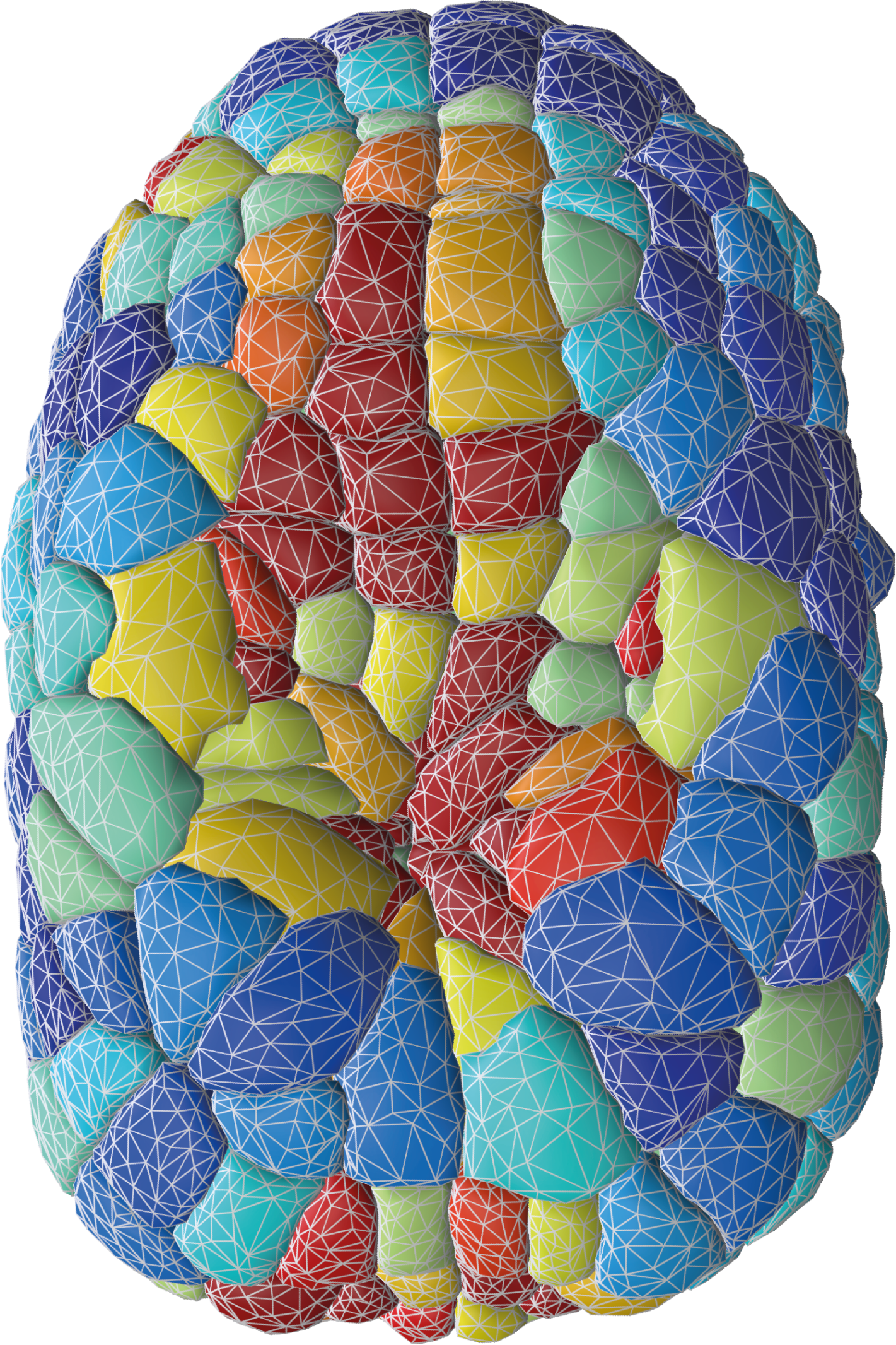
1. To segment membrane-labeled fluorescent images of embryos and tissues using an explicit triangle mesh representation of cell membrane interfaces. We recently released two computational approaches to precisely construct such a mesh:
2. To generate realistic fluorescent microscopy images from simulations with a user-defined microscope point-spread function (PSF). alphaMIC is a powerful CPU/GPU parallelised Python software that we designed to render artificial microscopy image generation from simulation meshes accessible, intuitive and fast. Coming with a handy user-interface and a Python back-end, it allows fast testing as well as easy integration in another Python pipeline. One can import its own PSF in the form of an image, or use already implemented theoretical PSFs such as Gaussian, Gibbson-Lanni or Wiener SIM. Images may be noised realistically in post-processing and time-series as well as 2D/3D meshes or different channels are intrinsically supported. We believe it may become one’s best companion to create the necessary datasets to compare, benchmark or train novel image analysis tools.
- delaunay-watershed is a Python package allowing one to reconstruct a non-manifold multimaterial triangle mesh from 3D segmentation masks of cells or nuclei. The method calculates the Euclidean distance transform of the masks to pave the image with a Delaunay tessellation. Applying watershed or equivalent segmentation algorithm on this irregular tessellation, ones recovers directly cell boundaries in the form of a mesh, either in 2D or 3D. Fast, versatile and avoiding arbitrary smoothing, it is the necessary step to create a very good first mesh of embryos or tissues for further geometric analysis.
- deltamic is a fully differentiable Python pipeline to render in 3D an artificial microscopy image from a triangle mesh. Exploiting the intrinsic automatic differentiation and GPU parallelisation of PyTorch, we apply our method to segment complex C. elegans embryo images by optimising simultaneously the shape of cells and the point-spread-function of an artificial microscope in order to best match the original microscopy image. This variational approach proves extremely precise and powerful, but at some computational cost. Our delaunay-watershed pipeline appears therefore key to have a very good first guess to start with and to converge fast.
MECHANICAL inference
We are working on novel mathematical and computation approaches to infer mechanical cell properties directly from membrane-labeled 3D microscopy of embryos.
More to come soon…
Collaborations: A. McDougall & R. Dumollard (LBDV)
3. Virtual Embryo : an integrated computational platform for data-driven 4D simulations
Collaborations: J. Bleyer (Ponts et Chaussées)
Collaborations: C. Patrat (Cochin Paris Hospital, APHP)
E. Labrune (Lyon public hospital, HCL)
We are developing an integrated framework based on C++ and Python to analyze and simulate the development of early embryos and tissues in 3D+time.
FINITE ELEMENTS for CELL and TISSUE morphogenesis
We are working on novel finite-element methods to simulate coupled bulk-surface problems arising in cell and tissue morphogenesis.
More to come soon…
Human EMBRYo and In Vitro Fertilization
We are working on novel artificial-intelligence approaches to help select embryo(s) with the highest chance to implant in medically assisted reproductive medicine techniques (IVF).
More to come soon…